



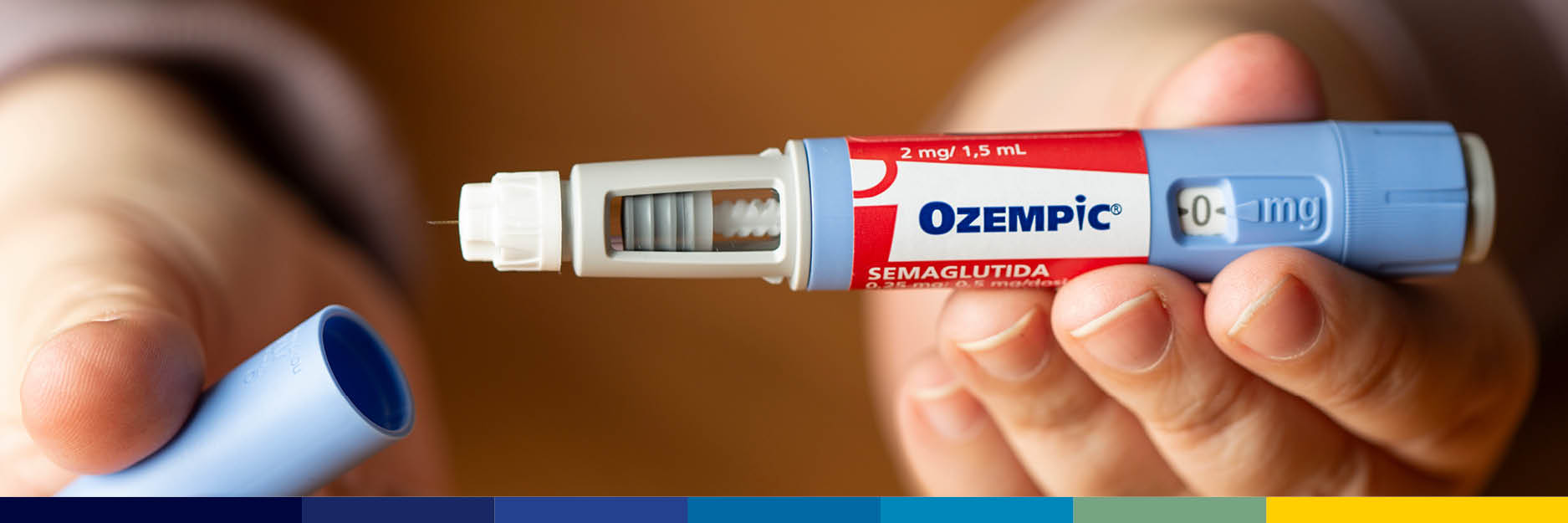
On October 2, 2024, Mylan Pharmaceuticals and Novo Nordisk reached a confidential settlement agreement regarding the launch date of generic versions of Ozempic (semaglutide). Although the agreement remains confidential…

The 2022 Inflation Reduction Act (IRA) makes significant changes to the Medicare Part D standard benefit, including capping annual enrollee out-of-pocket spending. The new provisions are being implemented gradually through 2025. While some changes…

On September 27th, 2024, the FDA approved Sanofi/Regeneron's Dupixent® (dupilumab) for use as an add-on maintenance treatment of adults who have inadequately controlled chronic obstructive pulmonary disease (COPD) and eosinophilic phenotype. In clinical trials,…

On October 2, 2024, the Food and Drug Administration (FDA) announced that it removed tirzepatide injection, a glucagon-like peptide 1 receptor agonist (GLP-1 RA) marketed by Eli Lilly, from its drug shortages list. Tirzepatide is the…

On September 20th, 2024, the U.S. Food and Drug Administration (FDA) approved MedImmune and AstraZeneca's FluMist® (influenza virus vaccine) for self-administration. FluMist® is…





